iso pipette calibration requirements|gilson pipette calibration requirements : factories ISO 8655, on the other hand, is a very detailed set of standards, methods and controls that a pipette calibration provider must follow to correctly and accurately calibrate piston pipettes. The specifications of ISO 8655 are rigorous and define in detail exactly what is required for a valid, trustworthy and reliable pipette calibration.
ASC Process Systems is the world's leading manufacturer of autoclaves and ovens for the aerospace, composites, nuclear, vulcanizing, and glass industries. We have been in business for over 30 years and are the recognized leader in autoclave and control system technology.
{plog:ftitle_list}
We’ve created this handy chamber cleaning guide — including a brief video tutorial — to prevent damage from happening and to ensure that your autoclave continues to operate as it should. How Often Should I Clean My Autoclave Chamber?

pipette volume calibration
The ISO 8655 covers the standards for calibrating and testing pipettes and other piston-operated volumetric devices. Information in the ISO 8655 includes standards for maximum permitted errors, guidelines for how to test and calibrate pipettes, reporting requirements to the end user, and .ISO (the International Organization for Standardization) is a worldwide federation of national standards bodies (ISO member bodies). The work of preparing International Standards is normally carried out . — metrological performance requirements for pipette tips have been further specified; . independent calibration, testing, verification .Updated: August 28, 2023 . Manufacturing medical devices is a highly complex process, and calibration requirements according to ISO 13485, Clause 7.6 (Control of monitoring and measuring equipment), mean high precision and close monitoring.Accuracy of all instruments decays with usage and wear and tear.ISO (the International Organization for Standardization) is a worldwide federation of national standards bodies (ISO member bodies). The work of preparing International Standards is normally carried out through ISO technical committees. Each member body interested in .
What are CAP Requirements Regarding Pipette Calibration? The answer is quite simple. . the three largest forces that affect the gravimetric test balances required for an ISO:8655 pipette calibration. Conclusion: As a best practice, the CAP Certified lab needs pipette calibrations performed in a controlled lab that operates using ISO:8655 .ISO 8655, on the other hand, is a very detailed set of standards, methods and controls that a pipette calibration provider must follow to correctly and accurately calibrate piston pipettes. The specifications of ISO 8655 are rigorous and define in detail exactly what is required for a valid, trustworthy and reliable pipette calibration.— general requirements for frequency of calibration, reporting measurement errors, exchangeable parts, metrological confirmation, . Pipettes; ISO 8655-3, Piston-operated volumetric apparatus — Part 3: Burettes; ISO 8655-4, Piston-operated volumetric apparatus — Part 4: Dilutors;
Remember to factor this requirement into your pipette calibration process to uphold the highest level of precision. Balances for Gravimetric Testing. ISO 8655:2022 imposes increased requirements on the balances used for gravimetric testing and calibration. For pipettes with a nominal volume of up to 20 μL, a 6-place balance is now necessary. This is why ISO 8655 is top standard for pipette calibration. ISO 8655 outlines extra requirements for the testing equipment, testing materials, and other environmental conditions. All elements of the testing procedures are recorded, and all the equipment is .This section specifies the procedure for determining the volume of a POVA using the gravimetric method, considered one of the gold standards for calibration. It defines strict requirements around equipment, pipette environmental conditions, and the number of test readings. Deviations are not permitted. • Part 7: Alternative measurement proceduresRevised ISO 8655 has ten parts with stricter guidelines about pipettes, errors, and user info. Pipette testing and calibration are in Parts 6, 7, and 8, with high-precision balance requirements. Sartorius has decoded the standard and shares its expertise on in-house or outsourcing pipette calibration, covering balances, pipettes, tips, and related services.
Pipette calibration and verification are compliant to ISO 8655. Check services and devices for calibration and routine testing of single and multichannel pipettes. . An XPR36/56 Microbalance with an evaporation trap fulfills ISO 8655 requirements for pipettes with nominal volumes as .ISO 8655 is the official standard for all piston operated volumetric apparatus. Our guide explains the 2022 pipette revisions and how to ensure compliance. . There are stricter requirements for calibration processes, including a minimum of 10 repeated measurements being required for compliance. . Routine testing is now required in addition .Learn how to choose the right pipette tips, comply with strict ISO 8655 standards, and distinguish between calibration and testing to ensure that your experiments are always on point. Tailored to the needs of laboratory professionals, this resource is a must-have for anyone looking to improve the quality of their research and data integrity.
ISO (International Organization for Standardization) is an independent, non-governmental organization with a membership of 162* national standards bod - ies. Through its members, ISO brings together experts to share knowledge and develop voluntary, consen - sus-based, market-relevant International Standardsor other externally regulated environment, a pipette’s calibration must be accurate, and must have an appropriate audit-trail. This is becoming increasingly important as laboratories apply for ISO accreditation, and also in light of the recent classification of pipettes as medical devices by the ISO standard authority,
ISO 8655:2022 contains requirements for producing and in-use control of piston-operated volumetric apparatus (POVA). This includes testing methods, testing environment, testing equipment, reporting requirements, requirements for measurement uncertainty, and general requirements for how POVAs work. The ISO 8655:2022 is applicable to pipettes,
Pipette.com offers ISO 17025 Onsite Pipette Calibration Services. Check the calibration of your pipettes regularly, depending on the frequency of use and on the application. Unmatched expertise in pipette calibration and repair. A2LA accredited, FDA registered.
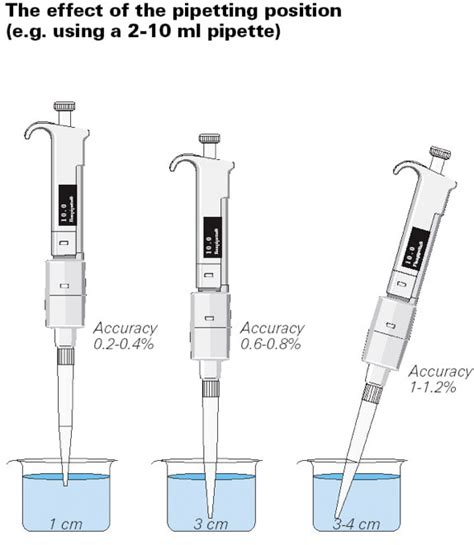
ISO 8655 is the official standard for all piston operated volumetric apparatus. Our guide explains the 2022 pipette revisions and how to ensure compliance. . There are stricter requirements for calibration processes, including a minimum of 10 repeated measurements being required for compliance. . Routine testing is now required in addition .
In the precise world of laboratory science, the accuracy and reliability of measurements are paramount. One of the most common tools for liquid handling is the pipette and ensuring its accuracy requires strict adherence to calibration standards. The International Organization for Standardization (ISO) has established such a guideline with the ISO .ISO 8655-6, but using the selected liquid instead of water. If the user readjusts the piston pipette, it shall be clearly and unequivocally indicated on the outside of the piston pipette that readjustment has been effected. The user shall mark the outside of the piston pipette with the name of the liquid for which the nominal volume now applies.Special requirements of ISO 8655 ISO 8655 standard describes many requirements that end-users and independent test-house operators should follow to fulfil the standard. These requirements can be classified under different categories. . Results from multichannel pipette calibration can be reported for two or more individual channels. These . But, when the pipette calibration service provider adheres to ISO 8655 standards and controls, the auditor evaluates the standards as ‘more than expected’. For pipette manufacturers, conforming to ISO 8655 standards helps to showcase service integrity, which in turn, corroborates the trust and reliability of the drug development lab.
ISO/IEC 17025 Accreditation Explained ISO/IEC 17025 is the international standard for both calibration and testing laboratories. It was initially known as ISO/IEC guide 25; ISO/IEC 17025 was originally issued by the international organization for standardization in 2000.
pipette calibration tips
Pipette calibration and verification are compliant to ISO 8655. Check services and devices for calibration and routine testing of single and multichannel pipettes. . An XPR36/56 Microbalance with an evaporation trap fulfills ISO 8655 requirements for pipettes with nominal volumes as . At our pipette calibration facility, we take accuracy seriously. Fortunately, the International Organization for Standardization (ISO) does, too! . In order to meet ISO 8655 requirements, users must meet strict standards for maximum measurement uncertainties, maximum permissible errors, the methodology of calibration, lab conditions, and .
Regular calibration is essential to maintain accuracy and precision. ISO 8655 differs from ISO 17025, as the former focuses on calibration procedures for piston-operated volumetric apparatus (POVA), while the latter sets requirements for .Pipette calibration certificate service ensures pipettes maintain manufacturer specifications. Mettler Toledo Calibration is compliant in ISO/IEC 17025, ISO 8655 , ISO 8655-6, and ISO 8655. . Rainin Pipette Calibration Services ISO/IEC 17025, ISO 8655 Service Experts. Order. 800 472 4646 (800 4 RAININ) . The requirements vary slightly from .

adiponectin elisa assay kit
Find an autoclave or sterilizer that will sterilize all instruments. Distributes of Sterilizers & Autoclaves. On site sterilizer repairs! When you need an autoclave that will sterilize your .
iso pipette calibration requirements|gilson pipette calibration requirements